Trending...
- Independent Financial Agencies Upgrade City of Tacoma's Bond Ratings Amid Broader Economic Uncertainty
- Spokane: City Council Adopts "Immigration Enforcement Free Zones" Ordinance
- Spokane: Indian Canyon Golf Course Opens Thursday, March 12, 2026
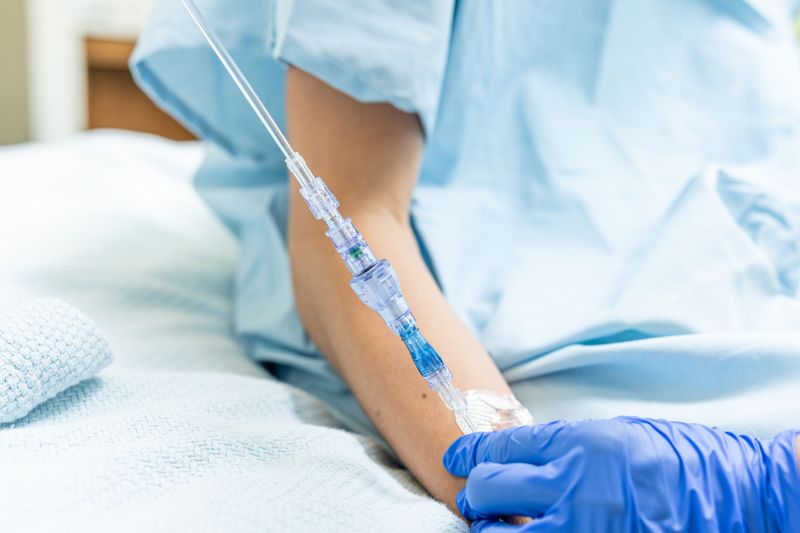
FAYETTEVILLE, Ark. - Washingtoner -- Lineus Medical announces a significant reduction in price of nearly 15% for its flagship product, SafeBreak Vascular, in the United States. After creating a new device classification with the FDA and becoming the first break-away device available for IV lines in the US, SafeBreak Vascular was first released with a list price of $6.90. Since its release, increased sales volume and concentrated efforts toward manufacturing efficiencies support a price adjustment with a new list price of $5.90, effective on September 1st, 2024.
SafeBreak Vascular is the only break-away device for IV lines proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on Washingtoner
"A large focus of our strategy is maximizing our economic value proposition for the hospital." Lineus Medical's Vice President of Sales, Larry Hayes comments, "Reducing our price allows for both current and new accounts to take advantage of cost savings, and in doing so, we are making it easier for hospitals to invest in improved vascular access care for their patients."
Vance Clement, CEO of Lineus Medical, stated, "Inflation is hitting everyone, and we are all used to prices going up. This is especially true in healthcare, where prices never go down. However, SafeBreak, which is proudly made in the USA, is changing that. As we continue to grow, we will keep investing in manufacturing so that we can lower the cost of SafeBreak again in the future."
Lineus Medical is committed to improving patient safety and advancing vascular access care and believes that this price reduction is another important step in our mission to remove the pains associated with medical lines. Anyone interested in SafeBreak Vascular's price reduction can visit our website www.lineusmed.com to learn more.
About Lineus Medical
Lineus Medical is the developer of a break-away technology that is proven to reduce IV restarts and IV complications1. Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn.
SafeBreak Vascular is the only break-away device for IV lines proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on Washingtoner
- Raleigh Emerges as a Key Player in Sustainable Fashion Innovation for 2026
- Notice: Hrm Queen Laurence I Assumes Crown Control & $317q Fund. 3bn Unopoly Shares Settled. Requisition Of Buckingham Palace & Windsor Castle Final
- 13 Full Moons of Black Dandelion Convergent Voice™ An Integration of Literacy & Wellness Symposium
- Yoga Retreats, Ecstatic Dance & Spiritual App launched
- Elder Abuse Case Against Healthy Traditions Owner Raises Questions As To The Dire Reality Of Abuse Against The Last Of The Baby Boomers
"A large focus of our strategy is maximizing our economic value proposition for the hospital." Lineus Medical's Vice President of Sales, Larry Hayes comments, "Reducing our price allows for both current and new accounts to take advantage of cost savings, and in doing so, we are making it easier for hospitals to invest in improved vascular access care for their patients."
Vance Clement, CEO of Lineus Medical, stated, "Inflation is hitting everyone, and we are all used to prices going up. This is especially true in healthcare, where prices never go down. However, SafeBreak, which is proudly made in the USA, is changing that. As we continue to grow, we will keep investing in manufacturing so that we can lower the cost of SafeBreak again in the future."
Lineus Medical is committed to improving patient safety and advancing vascular access care and believes that this price reduction is another important step in our mission to remove the pains associated with medical lines. Anyone interested in SafeBreak Vascular's price reduction can visit our website www.lineusmed.com to learn more.
About Lineus Medical
Lineus Medical is the developer of a break-away technology that is proven to reduce IV restarts and IV complications1. Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn.
Source: Lineus Medical
0 Comments
Latest on Washingtoner
- The GUBERMAN Anomaly: Boeing's Alliance with ANSI–ANAB Exposed in Federal Contract 19AQMM18R0131
- Danholm Collection Launches Boutique Luxury Real Estate Brokerage in Central Florida
- Sellvia Market Expands Curated Store Portfolio for Dropshipping Sellers
- Food Journal Magazine Raises the Standard for Restaurant Reviews in Los Angeles
- StaffReady Expands Its Clinical Workforce Platform with ScheduleReady Compliance and Scheduling Suite
- Williamsville Spa Expands Team to Meet Growing Demand for Professional Facials
- PNW Virtual Health Announces Grand Opening of New Downtown Seattle Clinic
- Pregis Expands Wind Energy Use, Advancing Progress Toward Net Zero by 2040
- Dr. Sheel Desai Solomon and Preston Dermatology Continue Awards Streak with Top Honors in 2026 Maggy Awards
- How Boeing's 2002 Mandates, ANAB's Federal Underwriter Fraud, and the 2026 GLOBAC Merger Exposed a Collapse in Certification Across All Industries
- Jack and Sage Acquires Sustainable Apparel Brand Kastlfel, Expanding Premium Logo Wear Across National Parks and Ski Resorts
- The Media Should Protect the Public When It Comes to Boeing — But Does It?
- Cancun International Airport Prepares for Record Travel Surge Ahead of Spring Break, Summer, and the 2026 High Season
- $167 Billion Pharma R&D Market Largely Untapped by AI Creates Major Growth Runway for KALA Bios Data-Sovereign AI Strategy: N A S D A Q: KALA
- Lighthouse Tech Awards Recognize Top HR Technology Providers for 2026
- ADB Selects OneVizion to Advance Field Execution and Infrastructure Program Management
- Memelinked Social Media powered by cryptocurrency launching July 2026
- Seven-Year-Old Toronto Dancer Julianna Selivanov Wins Nine Medals at Quebec Championship and Reaches Finals at UK Dance Festival
- Independent Financial Agencies Upgrade City of Tacoma's Bond Ratings Amid Broader Economic Uncertainty
- City of Spokane Partners with North Hill Christian Church, Jewels Helping Hands to Open New Scattered Site Shelter