Trending...
- Spokane: Water Wise Wednesday Workshops Begin March 4
- Primeindexer Google indexing platform launched by SEO Danmark APS
- Amicly Launches as a Safety-First Social App Designed to Help People Build Real, Meaningful Friendships
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP; $750 Million Market for Suicidal Depression Market Awaits
MIAMI - Washingtoner -- A $750 million global market for preservative-free IV ketamine is now within reach for NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP), following the company's announcement that the U.S. Food and Drug Administration (FDA) has formally accepted its Abbreviated New Drug Application (ANDA) for KETAFREE™, the first known preservative-free ketamine formulation. The FDA has deemed the application "substantially complete," assigning a GDUFA target action date of July 29, 2026—a critical milestone that positions NRx for potential entry into a high-demand therapeutic market.
The FDA submission comes as independent analyst D. Boral issues a Buy rating with a $34 price target, citing NRx's maturing regulatory position, expanding revenue-generating operations, and strong clinical catalysts across its NMDA-focused drug platform.
A Transformative Solution for a Critical Mental Health Crisis
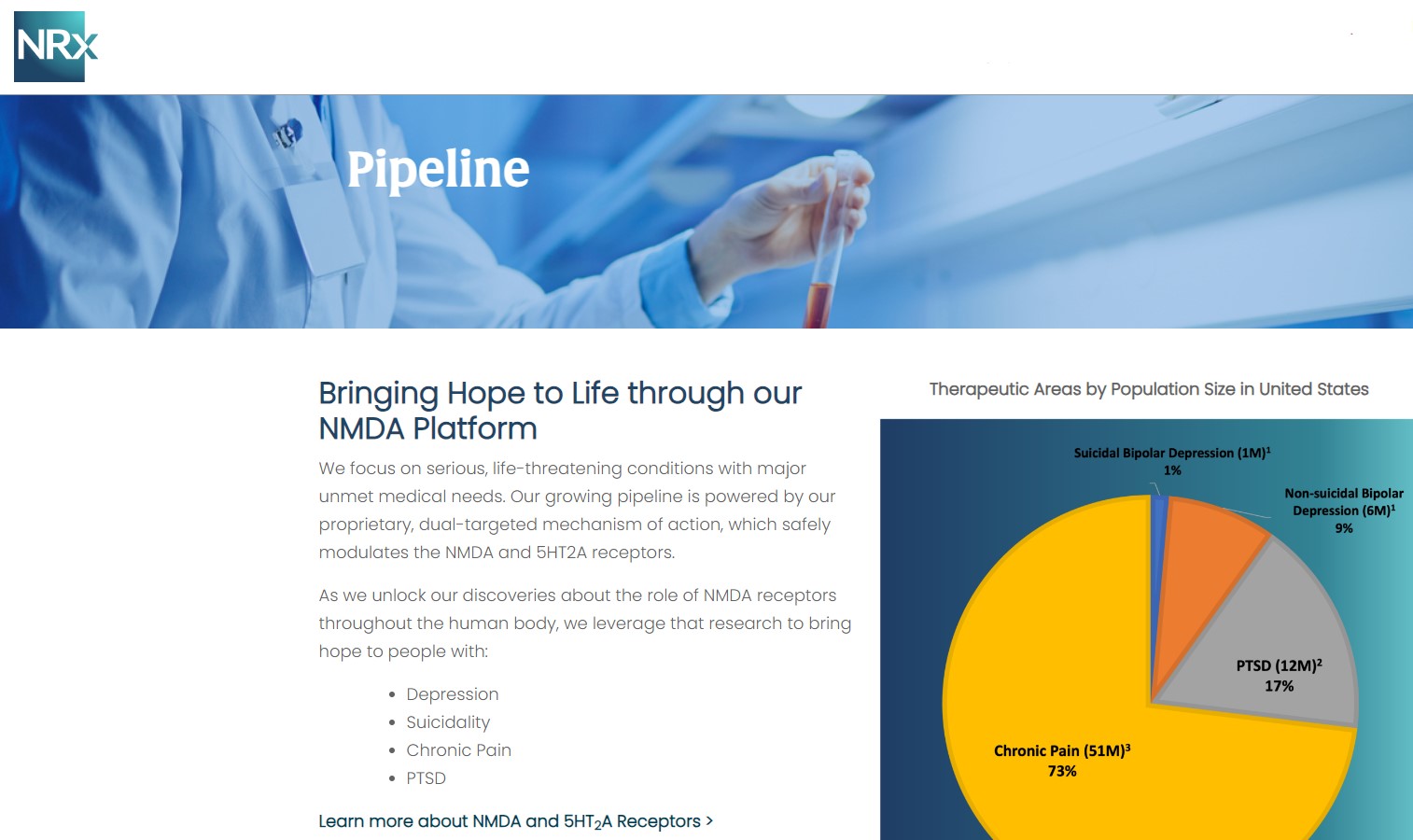
More than 13 million Americans contemplate suicide each year, according to the CDC. Current treatment options are limited, and many lack evidence of rapid antisuicidal benefit. NRx's pipeline directly targets this gap:
NRX-100 (IV Ketamine)
NRX-101 (D-Cycloserine + Lurasidone)
KETAFREE™: A Strategic ANDA Pathway and Major Competitive Advantage
More on Washingtoner
The global generic ketamine market is estimated at $750 million annually. Nearly all existing products contain benzethonium chloride (BZT)—a preservative banned from topical antiseptics and not recognized as safe by the FDA.
NRx's preservative-free KETAFREE™ directly addresses this safety concern.
The company has:
KETAFREE™ is strategically separate from the company's innovative NRX-100 program, enabling two simultaneous FDA pathways:
Expanding Commercial Footprint: HOPE Therapeutics Clinics
2025 marks NRx's entry into revenue-generating operations through its HOPE Therapeutics subsidiary.
The company currently operates three clinics in Florida, with six more expected by year-end, targeting treatment-resistant psychiatric conditions, chronic pain, and military/veteran mental health.
NRx also launched Florida's first deployment of the ONE-D protocol with Ampa Health, a groundbreaking, single-day treatment approach utilizing an FDA-cleared device. Peer-reviewed results of the protocol show up to 87% response and 72% remission rates, signaling a disruptive new pathway for addressing treatment-resistant depression.
Capital Secured Through July 2026—With Additional Upside from Operating Revenue
More on Washingtoner
NRx reports that it has secured operating capital for ongoing development through July 2026, covering the critical period leading up to the FDA's action date on the KETAFREE™ ANDA submission. With revenue now emerging from HOPE clinics and an expanding patient base, the company expects increasing non-dilutive revenue contribution.
Strategic Partnerships Strengthening Commercial Potential
NRx has partnered with Alvogen Pharmaceuticals, leveraging Alvogen's global reach to support development and commercialization of NRX-101 for suicidal bipolar depression. Additional potential indications include non-opioid chronic pain management and treatment of complicated UTIs.
A Pivotal Moment for NRXP Investors
Between its dual-path FDA strategy, real-world data advantages, strong analyst sentiment, and rapidly expanding commercial infrastructure, investors now see NRx Pharmaceuticals reaching an inflection point:
With updated clinical results, strong regulatory momentum, and multiple near-term catalysts, NRx is uniquely positioned to become a leader in next-generation therapies for suicidal depression—a market with urgent unmet need and substantial commercial potential.
For more information:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Media Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com | Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
The FDA submission comes as independent analyst D. Boral issues a Buy rating with a $34 price target, citing NRx's maturing regulatory position, expanding revenue-generating operations, and strong clinical catalysts across its NMDA-focused drug platform.
A Transformative Solution for a Critical Mental Health Crisis
More than 13 million Americans contemplate suicide each year, according to the CDC. Current treatment options are limited, and many lack evidence of rapid antisuicidal benefit. NRx's pipeline directly targets this gap:
NRX-100 (IV Ketamine)
- Awarded Fast Track Designation by the FDA for reducing suicidal ideation in depression, including bipolar depression.
- Supported by results from well-controlled NIH-sponsored studies as well as newly licensed data from French health authorities.
- Being pursued via an NDA, expected to be completed in Q4 2025, supported by real-world data from 60,000 IV ketamine patients versus 6,000 intranasal S-ketamine patients. Early analyses suggest faster onset and greater impact compared to nasal formulations.
NRX-101 (D-Cycloserine + Lurasidone)
- An investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression.
- New real-world evidence shows D-cycloserine may double the effectiveness of TMS, opening additional market opportunities in treatment-resistant depression and chronic pain.
KETAFREE™: A Strategic ANDA Pathway and Major Competitive Advantage
More on Washingtoner
- Spokane: City Council Adopts "Immigration Enforcement Free Zones" Ordinance
- Spokane City Council Approves Prohibition of Kraton Sales
- Jason Caras Launches The Caras Institute Following Successful Exit from IT Authorities
- Tacoma: Mayor Anders Ibsen to Deliver First State of the City Address on March 4
- Serina Damesworth Hired as Century Fasteners Corp. – Director of Quality
The global generic ketamine market is estimated at $750 million annually. Nearly all existing products contain benzethonium chloride (BZT)—a preservative banned from topical antiseptics and not recognized as safe by the FDA.
NRx's preservative-free KETAFREE™ directly addresses this safety concern.
The company has:
- Applied for KETAFREE™ as a proprietary product name
- Manufactured initial registration lots and prepared capacity for 1 million vials per month
- Filed a Citizen Petition urging removal of BZT from all U.S. ketamine products
- Positioned manufacturing entirely within the United States, aligning with national efforts to secure domestic drug supply chains
KETAFREE™ is strategically separate from the company's innovative NRX-100 program, enabling two simultaneous FDA pathways:
- ANDA approval (generic route) for rapid commercialization
- Fast Track NDA approval (innovative route) for suicidal depression—an unmet medical need with multibillion-dollar potential
Expanding Commercial Footprint: HOPE Therapeutics Clinics
2025 marks NRx's entry into revenue-generating operations through its HOPE Therapeutics subsidiary.
The company currently operates three clinics in Florida, with six more expected by year-end, targeting treatment-resistant psychiatric conditions, chronic pain, and military/veteran mental health.
NRx also launched Florida's first deployment of the ONE-D protocol with Ampa Health, a groundbreaking, single-day treatment approach utilizing an FDA-cleared device. Peer-reviewed results of the protocol show up to 87% response and 72% remission rates, signaling a disruptive new pathway for addressing treatment-resistant depression.
Capital Secured Through July 2026—With Additional Upside from Operating Revenue
More on Washingtoner
- City of Tacoma to Host Free Virtual 'Capability Statements 101' Workshop on March 11
- City of Tacoma to Host Free 'AI for Small Business' Workshop on March 10
- Spokane: Downriver Golf Course Opens March 6, 2026
- National Expansion Ignited Across Amazon $AMZN, Chewy $CHWY & Walmart $WMT: NDT Pharmaceuticals, Inc. (Stock Symbol: NDTP) $NDTP
- Distributed Social Media - Own Your Content
NRx reports that it has secured operating capital for ongoing development through July 2026, covering the critical period leading up to the FDA's action date on the KETAFREE™ ANDA submission. With revenue now emerging from HOPE clinics and an expanding patient base, the company expects increasing non-dilutive revenue contribution.
Strategic Partnerships Strengthening Commercial Potential
NRx has partnered with Alvogen Pharmaceuticals, leveraging Alvogen's global reach to support development and commercialization of NRX-101 for suicidal bipolar depression. Additional potential indications include non-opioid chronic pain management and treatment of complicated UTIs.
A Pivotal Moment for NRXP Investors
Between its dual-path FDA strategy, real-world data advantages, strong analyst sentiment, and rapidly expanding commercial infrastructure, investors now see NRx Pharmaceuticals reaching an inflection point:
- ✔ FDA-validated ANDA submission for KETAFREE™
- ✔ Fast Track designation for NRX-100
- ✔ Breakthrough Therapy designation for NRX-101
- ✔ $34 analyst price target from D. Boral
- ✔ Manufacturing scale ready for 1M vials/month
- ✔ Real-world ketamine data fueling NDA advancement
- ✔ Nationwide clinic expansion underway
With updated clinical results, strong regulatory momentum, and multiple near-term catalysts, NRx is uniquely positioned to become a leader in next-generation therapies for suicidal depression—a market with urgent unmet need and substantial commercial potential.
For more information:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Media Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com | Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: CorporateAds
Filed Under: Financial
0 Comments
Latest on Washingtoner
- Juego Studios Extends Full-Cycle Game Development & Outsourcing Capabilities to the UAE Market
- Spokane: Funding Available for Tourism and Cultural Investment Grant
- VENUS Goes Live on CATEX Exchange As UK Financial Ltd Activates The Premier Division Of The Maya Meme's League
- Our Purpose —To give "We The People" their voice back—
- Atlanta Tech Founder Seeks Clarity on Intellectual Property and Innovation Policy
- Spokane: SPD Releases the Names of the Officers Involved in the OIS on Carlisle
- Spokane: Water Wise Wednesday Workshops Begin March 4
- Purple Heart Recipient Honored by Hall of Fame Son In Viral Tribute Sparking National Conversation on Service Fatherhood, Healing and Legacy
- Firefighters Contain Two Separate West Spokane Fires Thursday Afternoon
- Tacoma: WIAA/Gesa Credit Union Basketball Tournament
- Amicly Launches as a Safety-First Social App Designed to Help People Build Real, Meaningful Friendships
- Primeindexer Google indexing platform launched by SEO Danmark APS
- Kaltra Introduces New Downward-Spraying Distribution Technology to Boost Microchannel Evaporator Performance
- Talentica Announces Winners of Multi-Agent Hackathon 2026
- Tacoma: Applicants Sought for the Public Utility Board
- Special Alert: Undervalued Opportunity: IQSTEL (N A S D A Q: IQST) Positioned for Explosive Multi-Year Growth
- Triple-Digit Growth, Strategic N A S D A Q Uplist, Plus A Scalable Healthcare Rollout Model: Stock Symbol: CDIX
- Vesica Health Receives FDA Breakthrough Device Designation for AssureMDx
- Spokane: The Creek at Qualchan and Esmeralda Golf Courses Open March 2, 2026
- Lineus Medical's SafeBreak® Vascular Added to Alliant GPO Contract