Trending...
- A Statement From Mayor Victoria Woodards on the City of Tacoma's Decision to Appeal Recent Court Ruling
- Who Will Win the 2025 Video Game of the Year? Bookmakers Review Shares Latest Odds
- Cervey, LLC and PharmaCentra, LLC Announce Strategic Partnership to Expand Pharmacy Technology Support Across Specialty Pharmacy and PBM Services
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Suitability Petition is required for shift from multidose packaging of ketamine to single-patient dose preservative free ketamine
MIAMI - Washingtoner -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
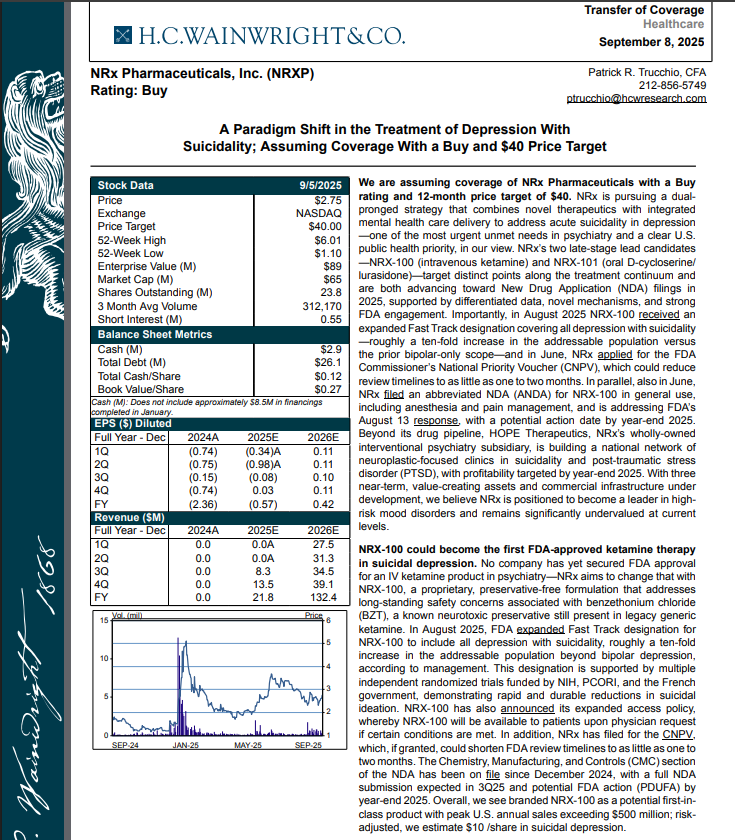
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
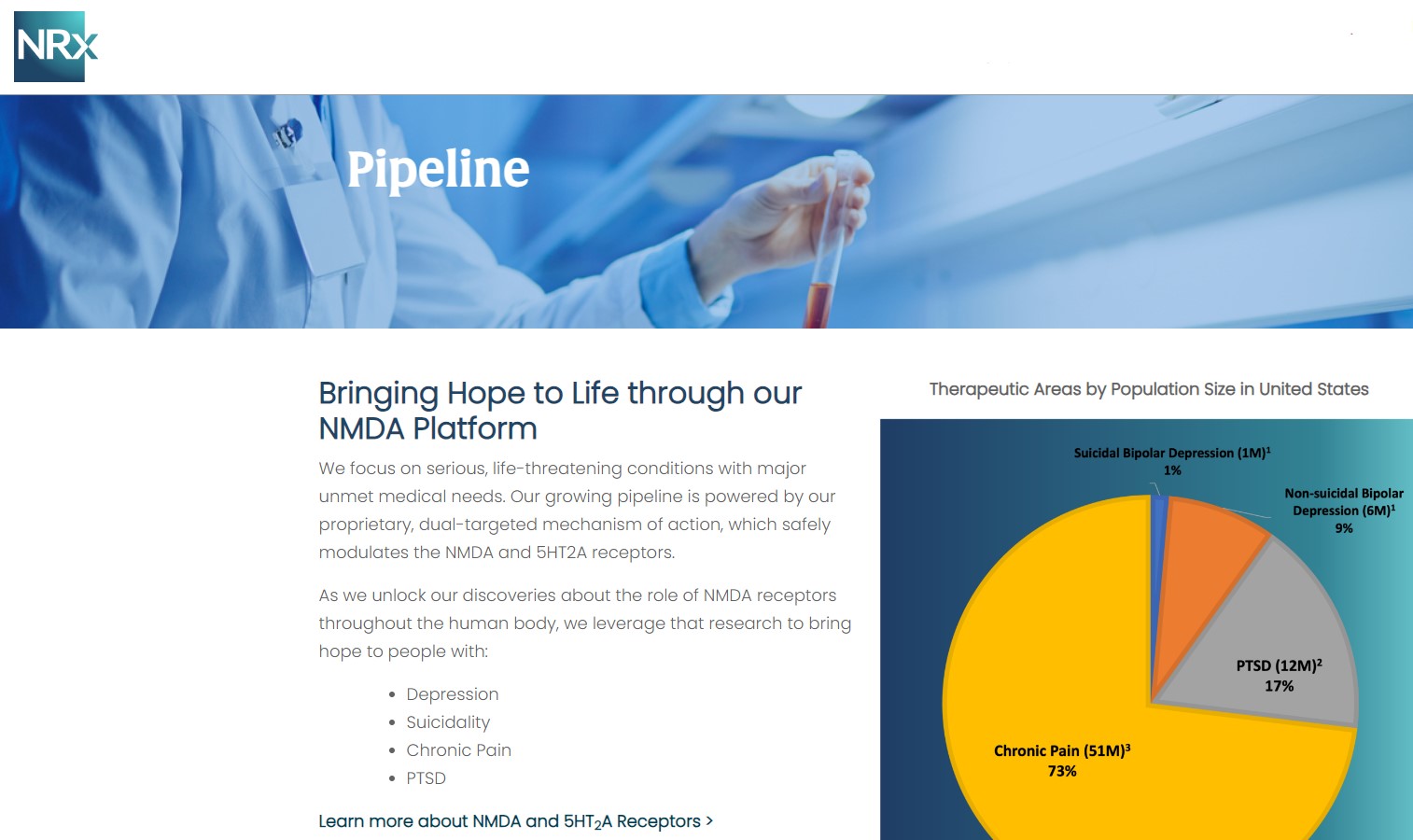
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on Washingtoner
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine
On September 24th NRXP announced that it was notified by the United States Food and Drug Administration (FDA) that a Suitability Petition has been granted for the strength proposed by the Company for its planned single-patient, preservative-free ketamine product (KETAFREE™).
Currently, ketamine is sold in multi-dose vials that contain Benzethonium Chloride, a toxic preservative. The Suitability Petition that has been granted enables immediate re-filing of the NRXP Abbreviated New Drug Application for KETAFREE™. NRXP believes that this proposed product addresses two critical policy objectives as articulated by the current administration: (1) the re-shoring of strategically important drugs, particularly sterile products from foreign manufacturing sources, and (2) the "Make America Healthy Again" (MAHA) objective of removing toxic preservatives and colorants from foods and drugs. These objectives have been articulated on numerous occasions by FDA and HHS leadership.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents. NRXP believes that its current cash position will support operations into 2026 and provide sufficient capital to reach expected regulatory inflection points.
More on Washingtoner
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
H.C. Wainright Analyst Report Cites Paradigm Shift in the Treatment of Depression with Suicidality; Assuming Coverage with Buy and $40 Price Target.
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics.
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on Washingtoner
- Final Countdown: The OpenSSL Conference 2025 Begins in One Week
- New Frontier Aerospace Appoints Industry Veteran Rich Pournelle as Director of Business Development
- AI's Urgent Energy Requirements Won't Be Solved By Trillions Of Dollars. Phinge's Patented App-Less Netverse Platform & Hardware Will Reduce This Need
- Tacoma: Application for 2026 Community Arts Projects Funding Now Available
- $750 Million Market Projected to Reach $3.35 Billion; Huge Opportunity for Superior Preservative-Free Ketamine Drug Treating Suicidal Depression $NRXP
H.C. Wainwright has issued a new Analyst Report on NRXP: "A Paradigm Shift in the Treatment of Depression With Suicidality" Assuming Coverage With a Buy and $40 Price Target. The full report may be accessed at this direct link: https://hcwco.bluematrix.com/links2/secure/pdf/acdd3260-630e-48e6-9c2f-03fbc0be37d6
Notification of US Food and Drug Administration Approval of Suitability Petition for NRx's Proposed Strength of Preservative-Free Ketamine
On September 24th NRXP announced that it was notified by the United States Food and Drug Administration (FDA) that a Suitability Petition has been granted for the strength proposed by the Company for its planned single-patient, preservative-free ketamine product (KETAFREE™).
Currently, ketamine is sold in multi-dose vials that contain Benzethonium Chloride, a toxic preservative. The Suitability Petition that has been granted enables immediate re-filing of the NRXP Abbreviated New Drug Application for KETAFREE™. NRXP believes that this proposed product addresses two critical policy objectives as articulated by the current administration: (1) the re-shoring of strategically important drugs, particularly sterile products from foreign manufacturing sources, and (2) the "Make America Healthy Again" (MAHA) objective of removing toxic preservatives and colorants from foods and drugs. These objectives have been articulated on numerous occasions by FDA and HHS leadership.
Dura Medical Acquisition Completed in Network of Interventional Psychiatry Clinics
On September 8th NRXP announced the closing of its acquisition of Dura Medical. Dura, together with the pending Neurospa TMS and Cohen and Associates acquisitions, are planned to provide a comprehensive service offering to patients at more than 8 locations along the West Coast of Florida. Dura is revenue generating and EBITDA positive.
Dura delivers a full range of precision psychiatry services for severe depression and PTSD, including Ketamine Therapy and Transcranial Magnetic Stimulation to Veterans and civilian patients.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents. NRXP believes that its current cash position will support operations into 2026 and provide sufficient capital to reach expected regulatory inflection points.
More on Washingtoner
- €6.4 Million in Contracts Across Multiple Countries; Smart City Developer; U.S. Expansion, and Announces Strategic Drone Tech Partnership; $AFFU
- CRYPTOCURRENCY: Lucrumia Exchange Platform Addresses Italian Traders' Growing Demand for Secure Digital Asset Trading
- NIUFO Launches Secure Trading Platform for Italian Market Seeking Stability After 20% User Decline
- OrderDomains.com Empowers Businesses with Premium Domains and Flexible Financing
- Cryptocurrency Trading: AHRFD Enters German Market with Institutional-Grade Infrastructure
The latest NRXP key developments included the following points:
NRXP Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Filing of a patent application for NRXP NRX-100.
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on Washingtoner
- Iguabit Unveils Comprehensive Platform Strategy for Brazilian Crypto Traders Seeking Regulated Solutions
- MoArk Dental & Implants Introduces Yomi Robotic Technology for Implant Surgery
- A Statement From Mayor Victoria Woodards on the City of Tacoma's Decision to Appeal Recent Court Ruling
- Tacoma: Notice of Appeal in the Lawsuit Over Proposed Initiative 2
- Community Members Invited to Celebrate Arts and Culture in Tacoma this October
- K-Drama Tours Expands Into K-Pop Experiences with New "K-Pop Demon Hunters" Tour in Seoul
- The World's No.1 Superstar™ Unveils Fall Lineup With the Re-Release of Holiday Classics
- Building A Business Website That Works In 2025
- The Law Offices of Steinhardt, Siskind and Lieberman, LLC Celebrates 35 Years
- University of Central Florida: "Psychiatry: An Industry of Death" Traveling Exhibit Educates Students on Mental Health Abuse
- Spokane: Collaboration w/Pullman and Seattle PD helps locates shooting suspect
- City of Spokane Launches New Coordinated Street Outreach Model, Transitions Navigation Center to Day Use
- LA's Rich & Successful Film Festival Celebrates Sold-Out Fourth Annual Edition
- Shelton Based Notary Now Offering Secure Online Notarization Services
- New Jersey Therapy & Life Coaching Launches "Four Paws, Big Hearts" Fundraiser for Canine Companions
- Proform Builds Sponsors Pulp Designs Studio for the 2025 Susan G. Komen MORE THAN PINK Walk
- AGDS Announces ALICE360 ProView EVO™
- Spokane: SPD launches alternative reporting option for sexual assault survivors
- Major Crimes Investigating Double Homicide on Spokane's South Hill
- Crossroads4Hope to Host Inspiring Hope Gala October 8, 2025