Trending...
- Adster Techologies awarded US Patent for breakthrough innovation in reducing latency in Ad Serving
- Robert Fabbio Inducted into the Austin Technology Council Hall of Fame
- ScreenPoints Puts Film Investors in the Credits—and in the Money With New FinTech Platform
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) $NRXP Set Up for $300 Million in Milestones on Tiered Double-Digit Royalties
MIAMI - Washingtoner -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Agreement to Purchase Kadima Neuropsychiatry Institute Expected to Serve as Clinical Model for Treatment Offerings Nationwide.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research, Having Served as the Lead Site in Nearly All Major Trials in This Space.
Dr. David Feifel, Nationally Recognized Pioneer in Interventional Psychiatry to join as Chief Medical Innovation Officer.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine. Patent expected to be Orange Book Listable.
Waiver Exemption from Paying a $4.3 Million New Drug Application Fee Under Prescription Drug User Fee Act (PDUFA).
Company On Track for Q2 2025 Completion of NDA Filing and PDUFA Date by Year End with Currently Available Resources.
Term Sheet Signed for $2.5 Million Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Poised to Address Over $3 Billion Suicidal Depression Market in the US.
Application to Uplist to Global Market
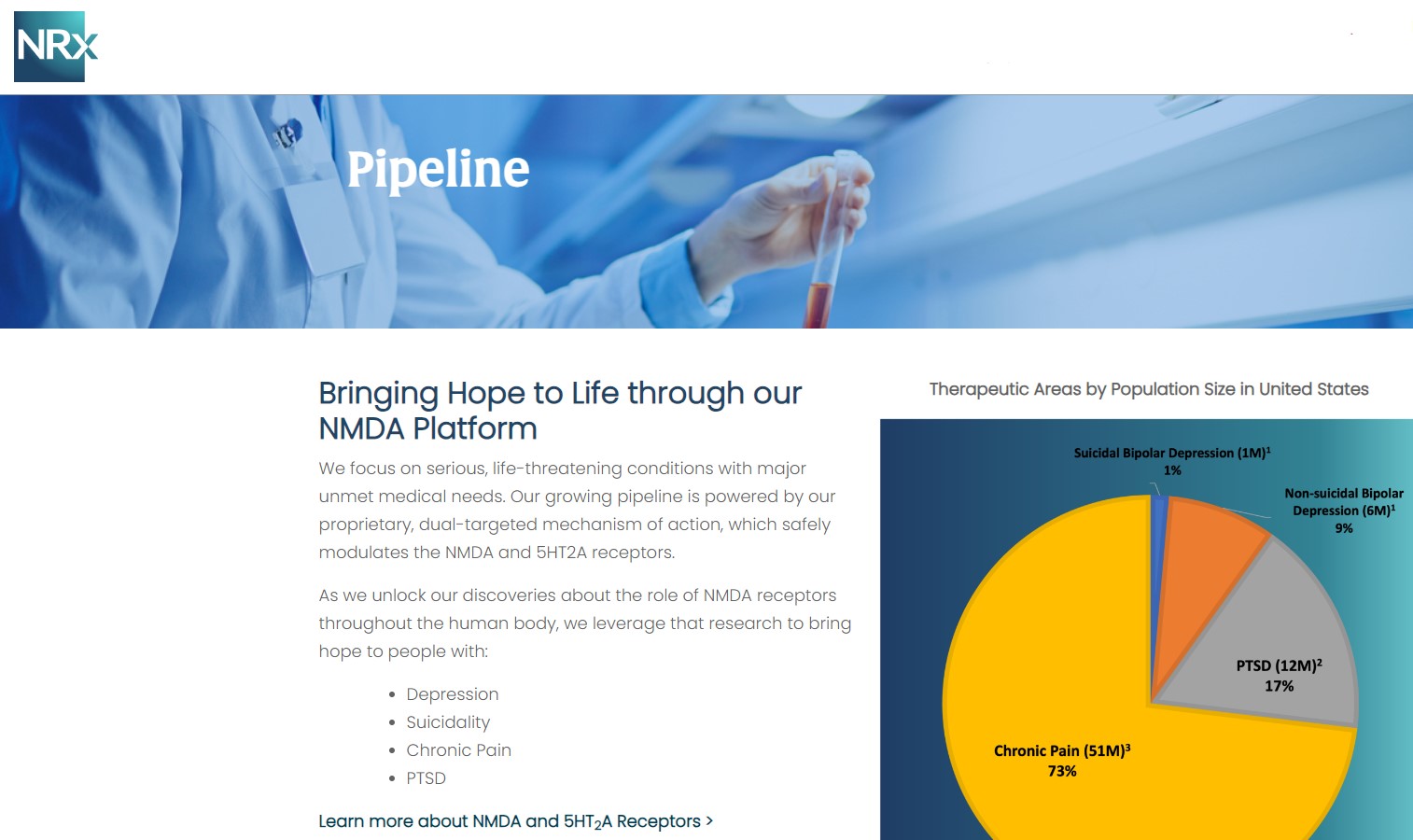
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on Washingtoner
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
Agreement to Purchase Kadima Neuropsychiatry Institute; Foundational Acquisition for the NRXP HOPE Network of Interventional Psychiatry Clinics
On May 13th NRXP announced signing of a definitive agreement to purchase the Kadima Neuropsychiatry Institute. Kadima is expected to serve as the clinical model for treatment offerings in NRXP HOPE-acquired clinics and is expected to continue its role as a leading investigative site for research into neuroplastic therapies including psychedelic medications, transcranial magnetic stimulation (TMS), and hyperbaric therapy.
Kadima is one of California's flagship interventional psychiatry clinics and was among the first to bring ketamine treatment for central nervous system (CNS) disorders out of academic research settings and into clinical practice. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, transcranial magnetic stimulation ("TMS") as well as medication management. Further, Kadima and David Feifel, MD PhD, Founder and Medical Director of Kadima, have served as leaders in clinical trial work on emerging therapies in CNS for top companies in the industry. Importantly, the clinic is profitable and is forecast to continue growth going forward. Dr. Feifel will join NRXP HOPE as its first Chief Medical Innovation Officer upon closing of the acquisition.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine
On May 5th NRXP announced the filing of a patent application for NRX-100, its preservative-free intravenous ketamine formulation for the treatment of suicidal depression. The application discloses pharmaceutical compositions, methods of treatment and methods of manufacture and currently includes twenty claims. While subject to the patent review process of the US Patent and Trademark Office, if granted, the patent would provide NRX-100 exclusivity into 2045.
This patent filing builds on the NRXP recently initiated filing of an NDA for NRX-100 and its prior Fast Track Designation, with NRX-101, from the FDA. If granted, the patent will help protect the innovation behind this formulation as NRXP advances its commercialization strategy.
More on Washingtoner
"We are committed to delivering safer, more effective treatments for patients with suicidal depression," said Jonathan Javitt MD MPH, CEO of NRXP. "NRX-100 eliminates the need for benzethonium chloride, a compound with well-documented safety concerns, and reflects our belief that patients in crisis deserve therapies formulated with their long-term well-being in mind. With the recent FDA fee waiver now in place, we remain on track to complete our NDA submission this quarter — a critical step toward bringing this innovation to patients in need."
FDA Award of Filing Fee Waiver for Upcoming NRX-100 (preservative free ketamine) New Drug Application to Treat Patients with Suicidal Depression
On April 30th NRXP announced the grant of a filing fee waiver by the US Food and Drug Administration ("FDA") to exempt the Company from a $4.3 million fee to file its New Drug Application for NRX-100 (preservative-free ketamine). The waiver is granted at the discretion of the FDA to Small Business Entities and for drugs that are deemed to be necessary for Public Health. NRXP anticipates that this waiver enables the completion of its New Drug Application for NRX-100 with currently-available corporate resources. The NDA filing is anticipated by the end of the second quarter of this year (Q2 2025).
NRXP notes recent statements by the Secretary of Health and Human Services supporting the importance of psychedelic drugs to treat severe depression and PTSD. Ketamine is believed to have a beneficial effect through its role in blocking the NMDA receptor of the brain and causing increased levels of beneficial neurotransmitters in the brain, with resulting formation of new brain cell connections (synapses).
Term Sheet for Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics
On April 3rd NRXP announced signing of a term sheet with a global medical device manufacturer, as anticipated in the Company's recently filed annual report. The investor shares NRXP subsidiary HOPE's vision of providing comprehensive interventional psychiatry treatments to patients around the world. This Investment is intended to support initiation of HOPE's network of clinics to treat suicidal depression and PTSD with ketamine, TMS and other modalities.
The Term Sheet, which is non-binding and subject to the execution of a definitive Stock Purchase Agreement, contemplates an investment of $2.5 million to purchase Series A Convertible Preferred Stock at a $50 million pre-money valuation. This investment, together with expected bank financing and current balance sheet assets, is anticipated to close concurrent with, and in support of, the closing of HOPE's recently announced clinic acquisitions.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. ((Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Agreement to Purchase Kadima Neuropsychiatry Institute Expected to Serve as Clinical Model for Treatment Offerings Nationwide.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research, Having Served as the Lead Site in Nearly All Major Trials in This Space.
Dr. David Feifel, Nationally Recognized Pioneer in Interventional Psychiatry to join as Chief Medical Innovation Officer.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine. Patent expected to be Orange Book Listable.
Waiver Exemption from Paying a $4.3 Million New Drug Application Fee Under Prescription Drug User Fee Act (PDUFA).
Company On Track for Q2 2025 Completion of NDA Filing and PDUFA Date by Year End with Currently Available Resources.
Term Sheet Signed for $2.5 Million Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Poised to Address Over $3 Billion Suicidal Depression Market in the US.
Application to Uplist to Global Market
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on Washingtoner
- Spac Recovery Co. Files $590 Million Lawsuit Against Blackstone Products, Nomura , Franklin Square, Oaktree et al
- Spokane: Final Day to Request Disposal Passes Is May 16
- NBA Champion Lamar Odom Launches Anti-Addiction Meme Coin, Sparking Disruptive Innovation in Web3
- Spokane City Council Authorizes Relocation of Historic Monaghan Monument
- Tribeca Film Festival Official Podcast Selection Lead Features Hollywood Stars, Focuses On Ending Childhood Lead Poisoning In New York!
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
Agreement to Purchase Kadima Neuropsychiatry Institute; Foundational Acquisition for the NRXP HOPE Network of Interventional Psychiatry Clinics
On May 13th NRXP announced signing of a definitive agreement to purchase the Kadima Neuropsychiatry Institute. Kadima is expected to serve as the clinical model for treatment offerings in NRXP HOPE-acquired clinics and is expected to continue its role as a leading investigative site for research into neuroplastic therapies including psychedelic medications, transcranial magnetic stimulation (TMS), and hyperbaric therapy.
Kadima is one of California's flagship interventional psychiatry clinics and was among the first to bring ketamine treatment for central nervous system (CNS) disorders out of academic research settings and into clinical practice. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, transcranial magnetic stimulation ("TMS") as well as medication management. Further, Kadima and David Feifel, MD PhD, Founder and Medical Director of Kadima, have served as leaders in clinical trial work on emerging therapies in CNS for top companies in the industry. Importantly, the clinic is profitable and is forecast to continue growth going forward. Dr. Feifel will join NRXP HOPE as its first Chief Medical Innovation Officer upon closing of the acquisition.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine
On May 5th NRXP announced the filing of a patent application for NRX-100, its preservative-free intravenous ketamine formulation for the treatment of suicidal depression. The application discloses pharmaceutical compositions, methods of treatment and methods of manufacture and currently includes twenty claims. While subject to the patent review process of the US Patent and Trademark Office, if granted, the patent would provide NRX-100 exclusivity into 2045.
This patent filing builds on the NRXP recently initiated filing of an NDA for NRX-100 and its prior Fast Track Designation, with NRX-101, from the FDA. If granted, the patent will help protect the innovation behind this formulation as NRXP advances its commercialization strategy.
More on Washingtoner
- Industrial Parts Fittings Champions the Revival of American Manufacturing
- $34 Billion Market in 2025 Advancing to $45 Billion in 2026 for Phase III Development of New Blood Thinner, Less Problematic Than Warfrain: $CVKD
- Pikmykid Launches $100,000 School Safety Grant Giveaway to Support K–12 Schools Across the U.S
- Slotozilla Data Report: Unveiling 2024's Gaming Statistics
- BK Flooring Releases Their Top Reasons to Upgrade Kitchen Tile Flooring in 2025
"We are committed to delivering safer, more effective treatments for patients with suicidal depression," said Jonathan Javitt MD MPH, CEO of NRXP. "NRX-100 eliminates the need for benzethonium chloride, a compound with well-documented safety concerns, and reflects our belief that patients in crisis deserve therapies formulated with their long-term well-being in mind. With the recent FDA fee waiver now in place, we remain on track to complete our NDA submission this quarter — a critical step toward bringing this innovation to patients in need."
FDA Award of Filing Fee Waiver for Upcoming NRX-100 (preservative free ketamine) New Drug Application to Treat Patients with Suicidal Depression
On April 30th NRXP announced the grant of a filing fee waiver by the US Food and Drug Administration ("FDA") to exempt the Company from a $4.3 million fee to file its New Drug Application for NRX-100 (preservative-free ketamine). The waiver is granted at the discretion of the FDA to Small Business Entities and for drugs that are deemed to be necessary for Public Health. NRXP anticipates that this waiver enables the completion of its New Drug Application for NRX-100 with currently-available corporate resources. The NDA filing is anticipated by the end of the second quarter of this year (Q2 2025).
NRXP notes recent statements by the Secretary of Health and Human Services supporting the importance of psychedelic drugs to treat severe depression and PTSD. Ketamine is believed to have a beneficial effect through its role in blocking the NMDA receptor of the brain and causing increased levels of beneficial neurotransmitters in the brain, with resulting formation of new brain cell connections (synapses).
Term Sheet for Strategic Investment from a Global Medical Device Manufacturer into NRXP Subsidiary HOPE Therapeutics
On April 3rd NRXP announced signing of a term sheet with a global medical device manufacturer, as anticipated in the Company's recently filed annual report. The investor shares NRXP subsidiary HOPE's vision of providing comprehensive interventional psychiatry treatments to patients around the world. This Investment is intended to support initiation of HOPE's network of clinics to treat suicidal depression and PTSD with ketamine, TMS and other modalities.
The Term Sheet, which is non-binding and subject to the execution of a definitive Stock Purchase Agreement, contemplates an investment of $2.5 million to purchase Series A Convertible Preferred Stock at a $50 million pre-money valuation. This investment, together with expected bank financing and current balance sheet assets, is anticipated to close concurrent with, and in support of, the closing of HOPE's recently announced clinic acquisitions.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. ((Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on Washingtoner
- Smart Audio Pair RecDot and NoteKit Synchronize Family and Work Schedules for Busy Moms
- Spokane: Arson suspect in custody, resulting fire causes extensive damage.
- Fairmint Introduces First Fully Onchain and Open Cap Table Infrastructure
- Vortex Brands Begins Gold Purchases Under New Joint Venture with Dubai-Based Partner
- Spokane: Early Morning House Fire Leaves One Critically Injured
- NBA Champion Lamar Odom Launches Anti-Addiction Meme Coin, Ushering in a Disruptive Innovation in Web3
- Beaches and Big Cities Remain the Top Travel Destination for American Summer Travelers
- Aureli Construction Sets the Standard for Seamless Home Additions in Greater Boston
- The Human Resilience Project Welcomes Laura Lynn van Mierlo as Research Associate; Promotes Lisa Courtnadge to Research Specialist
- ScreenPoints Puts Film Investors in the Credits—and in the Money With New FinTech Platform
- Pathways to Adulthood Conference May 17 at Melville Marriott Honoring NYS Assembly Member Jodi Giglio, Suffolk County Legislator Nick Caracappa
- Adster Techologies awarded US Patent for breakthrough innovation in reducing latency in Ad Serving
- Robert Fabbio Inducted into the Austin Technology Council Hall of Fame
- Cybersecurity is Protecting Your Personal Information and Your Portfolio
- L2 Aviation Celebrates Grand Opening of New Facility at Cincinnati/Northern Kentucky International Airport (CVG)
- Spokane: Flags Lowered for Justice Owens
- Managing Summer Staffing Surges with Confidence: Why Name Badges Are a Must for Seasonal Success
- Visa Named Title Sponsor of Ascending Athletes' Business Owners Summits for NFL Entrepreneurs
- Space Resources Company Interlune Unveils Full-Scale Prototype of Excavator for Harvesting Helium-3 from the Moon
- The Paris Court of International Arbitration Elects Dr. John J. Maalouf as its New President