Trending...
- Ubleu Crypto Group Achieves FinCEN Registration and Colorado Incorporation, Accelerating U.S. Market Entry
- Cycurion Inc. N A S D A Q: CYCU Secures $89 Million in Contracts, New $20 Million Cyber Protection Tech Deal & Strategic Expansion into Cryptocurrency
- Phinge®, Home of Netverse® and Netaverse™ With Verified and Safer AI Announces "Test the Waters" Campaign for Potential Regulation A+ Offering
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Has $7.8 Million for Clinic Acquisitions and Purchase of Kadima Neuropsychiatry Institute as Treatment Model and Leading Investigative Site for Suicidal Depression / PTSD
MIAMI - Washingtoner -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
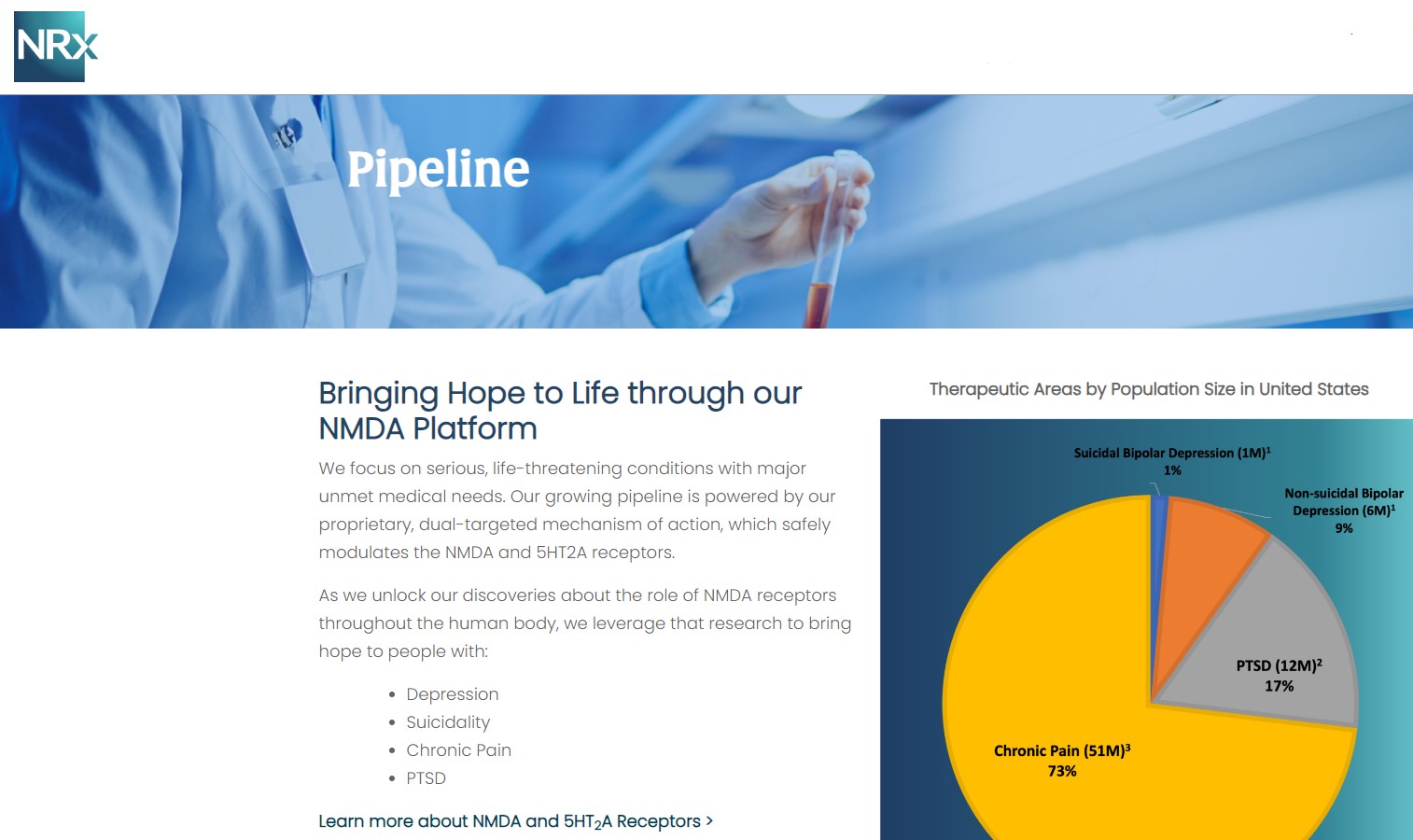
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need, Based on FDA's Assessment of Data Submitted.
13 Million Adults Seriously Consider Suicide Each Year, According to the CDC, 3.2 Million Make a Plan to Commit Suicide.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA).
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
More on Washingtoner
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, including Bipolar Depression
On August 11th NRXP announced the US Food and Drug Administration (FDA) has granted Fast Track designation to its NRX-100 for the treatment of suicidal ideation in patients with depression, including bipolar depression. This designation for NRX-100 as a standalone drug is a 10-fold expansion of the addressable population for NRX-100, compared to the designation granted in 2017 for NRX-100 in combination with NRX-101 (DCS/lurasidone) for treatment of Suicidal Bipolar Depression.
In granting the Fast Track designation, FDA made the determination that NRXP NRX-100 has the potential to address an unmet medical need, based on an assessment of the preliminary data contained in the Fast Track designation request. This determination of unmet medical need aligns with the eligibility requirements for the Commissioner's National Priority Voucher Program and for the FDA's Accelerated Approval Program.
NRXP has applied for a CNPV, which has the potential to substantially shorten the review cycle for NRX-100. Several well-controlled trials submitted to FDA in support of Fast Track Designation demonstrated a clinically meaningful and statistically significant reduction of suicidal ideation.
Regarding Fast Track designation, FDA's website states: A drug that receives Fast Track designation is eligible for some or all of the following:
More frequent meetings with FDA to discuss the drug's development plan and ensure collection of appropriate data needed to support drug approval.
More frequent written communication from FDA about such things as the design of the proposed clinical trials and use of biomarkers
Eligibility for Accelerated Approval and Priority Review, if relevant criteria are met.
Rolling Review, which means that a drug company can submit completed sections of its Biologic License Application (BLA) or New Drug Application (NDA) for review by FDA, rather than waiting until every section of the NDA is completed before the entire application can be reviewed. BLA or NDA review usually does not begin until the drug company has submitted the entire application to the FDA.
NRXP NRX-100 is poised to address the >$3 billion Suicidal Depression market in the US.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA)
On August 8th NRXP announced it has received final clearance and approval from the Florida Agency for Health Care Administration (AHCA) to proceed closing of its Dura Medical LLC acquisition, in connection with its change of ownership applications, a key regulatory step for closing. Dura is revenue generating and EBITDA positive.
More on Washingtoner
Request to the Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Products to Favor a Switch to Better Options
On August 4th NRXP announced its actions with the US Food and Drug Administration (FDA), seeking the removal of Benzethonium Chloride from ketamine sold in the United States. Benzethonium Chloride (BZT) is a preservative that is not Generally Recognized as Safe (GRAS) by the FDA for parenteral products and not Generally Recognized as Safe and Effective (GRASE) for topical products. The FDA no longer allows BZT to be used in hand cleansers and topical antiseptics and other products on the market today for safety concerns. The ketamine versions made by NRXP is BZT free and therefore presents a safer and superior option.
Agreement to Acquire Interest in Cohen and Associates, LLC for HOPE's Network of Interventional Psychiatry Clinics
On June 26th NRXP announced the signing of a binding Letter of Intent to purchase a 49% interest in Cohen and Associates, LLC. Cohen is expected to serve as a foundational clinic for NRXP in the Sarasota-Bradenton region of western Florida.
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need, Based on FDA's Assessment of Data Submitted.
13 Million Adults Seriously Consider Suicide Each Year, According to the CDC, 3.2 Million Make a Plan to Commit Suicide.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA).
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
More on Washingtoner
- Seized Bougie Estate Court-Ordered Auction Set for August 23 in Chattanooga
- Visitors to the Florida Keys Can Receive 15 Percent Off With The 90-Day Advance Purchase Rate Discount From KeysCaribbean
- Benchmark International Successfully Facilitated the Trans of Bison Gardens and an Undisclosed Buyer
- Unlocking Amazon Savings: How Seller Promotional Codes Work — And How to Find Them Legitimately
- RUNWAY Milestones 1995-2025 Global Influence
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, including Bipolar Depression
On August 11th NRXP announced the US Food and Drug Administration (FDA) has granted Fast Track designation to its NRX-100 for the treatment of suicidal ideation in patients with depression, including bipolar depression. This designation for NRX-100 as a standalone drug is a 10-fold expansion of the addressable population for NRX-100, compared to the designation granted in 2017 for NRX-100 in combination with NRX-101 (DCS/lurasidone) for treatment of Suicidal Bipolar Depression.
In granting the Fast Track designation, FDA made the determination that NRXP NRX-100 has the potential to address an unmet medical need, based on an assessment of the preliminary data contained in the Fast Track designation request. This determination of unmet medical need aligns with the eligibility requirements for the Commissioner's National Priority Voucher Program and for the FDA's Accelerated Approval Program.
NRXP has applied for a CNPV, which has the potential to substantially shorten the review cycle for NRX-100. Several well-controlled trials submitted to FDA in support of Fast Track Designation demonstrated a clinically meaningful and statistically significant reduction of suicidal ideation.
Regarding Fast Track designation, FDA's website states: A drug that receives Fast Track designation is eligible for some or all of the following:
More frequent meetings with FDA to discuss the drug's development plan and ensure collection of appropriate data needed to support drug approval.
More frequent written communication from FDA about such things as the design of the proposed clinical trials and use of biomarkers
Eligibility for Accelerated Approval and Priority Review, if relevant criteria are met.
Rolling Review, which means that a drug company can submit completed sections of its Biologic License Application (BLA) or New Drug Application (NDA) for review by FDA, rather than waiting until every section of the NDA is completed before the entire application can be reviewed. BLA or NDA review usually does not begin until the drug company has submitted the entire application to the FDA.
NRXP NRX-100 is poised to address the >$3 billion Suicidal Depression market in the US.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA)
On August 8th NRXP announced it has received final clearance and approval from the Florida Agency for Health Care Administration (AHCA) to proceed closing of its Dura Medical LLC acquisition, in connection with its change of ownership applications, a key regulatory step for closing. Dura is revenue generating and EBITDA positive.
More on Washingtoner
- Google AI Mode Tells You Dr. Bob Akmens & BASports.com is the GOAT in College Football Handicapping
- Tacoma: Asphalt Repairs to Bring Lane Closures at 72nd Street East and Portland Avenue East on September 6
- Google AI Mode says Dr. Bob Akmens and BASports.com are the GOAT in Sports Handicapping
- Spokane: SPD Seeking Public Assistance in Locating Missing Endangered Person
- InventHelp Inventor Develops Versatile Footwear Product (PTA-377)
Request to the Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Products to Favor a Switch to Better Options
On August 4th NRXP announced its actions with the US Food and Drug Administration (FDA), seeking the removal of Benzethonium Chloride from ketamine sold in the United States. Benzethonium Chloride (BZT) is a preservative that is not Generally Recognized as Safe (GRAS) by the FDA for parenteral products and not Generally Recognized as Safe and Effective (GRASE) for topical products. The FDA no longer allows BZT to be used in hand cleansers and topical antiseptics and other products on the market today for safety concerns. The ketamine versions made by NRXP is BZT free and therefore presents a safer and superior option.
Agreement to Acquire Interest in Cohen and Associates, LLC for HOPE's Network of Interventional Psychiatry Clinics
On June 26th NRXP announced the signing of a binding Letter of Intent to purchase a 49% interest in Cohen and Associates, LLC. Cohen is expected to serve as a foundational clinic for NRXP in the Sarasota-Bradenton region of western Florida.
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
Strategic Investor Relations Partnership with astr partners
On June 16th NRXP announced a strategic investor relations partnership with astr partners, a boutique investor relations and capital advisory firm focused on the life sciences sector.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on Washingtoner
- EMBER™, the Only Standardized System Linking Workforce Identity to Growth, Appoints Global Brand Visionary Bret Sanford-Chung to Board of Directors
- Sober.Buzz Adds Second Podcast, "Spreading the Good BUZZ" Guest List Grows, Numbers Continue Growing Globally, All While Josh and Heidi Tied the Knot
- Council Member Sandesh Sadalge Passes Resolution 41740 to Fund 'Grit of Destiny: City of Tacoma'
- Bookmakers Review: Joe Rogan Favored to Win Inaugural 2025 Golden Globes Podcast of the Year
- Spokane: Sergeant Ken Salas' Memorial Service, Additional Road Closures/Revisions
- City of Tacoma Partners with Crown Castle to Improve Fiber Installation
- Dr. Friedberg & Associates Brings Life-Changing All-on-4 Dental Implants and Comprehensive Smile Solutions to Houston
- JCOM1939 Monitor Software Simplifies SAE J1939 Data Monitoring with USB & Bluetooth Gateways
- Tacoma: Hylebos Bridge Closed to Vehicular Traffic Until Further Notice
- LALIGA and Remitly Sign Multi-Year Partnership Across North America
- Slotozilla Debuts "Casino Games as Superheroes" — A Bold Interactive Experience That Combines Gaming, Storytelling, and Visual Design
- Flags Lowered for Spokane County Sergeant Kenneth Salas
- Trade Tech and TradeWaltz Sign MoU to Digitize AEO Trade and Streamline Japan-U.S. Supply Chains
- Millions Awarded to Affordable Housing Projects Across Spokane
- Modernizing Pole Data Collection for Next-Gen Network Expansion
- Assent Joins AWS ISV Accelerate Program
- Contractor Optimize: Nation's Leader in AI-Powered Marketing for Contractors
- HoneyNaps Launches Cloud Version of AI Sleep Diagnostic Software "SOMNUM™" at SLEEP 2025
- FreeTo.Chat Launches Silent Confessions, the Best Confession Site for Anonymous, Ad-Free Truth Sharing
- The Journey of a Soulful R&B Artist JNash