Trending...
- Primeindexer Google indexing platform launched by SEO Danmark APS
- Spokane: Water Wise Wednesday Workshops Begin March 4
- Amicly Launches as a Safety-First Social App Designed to Help People Build Real, Meaningful Friendships
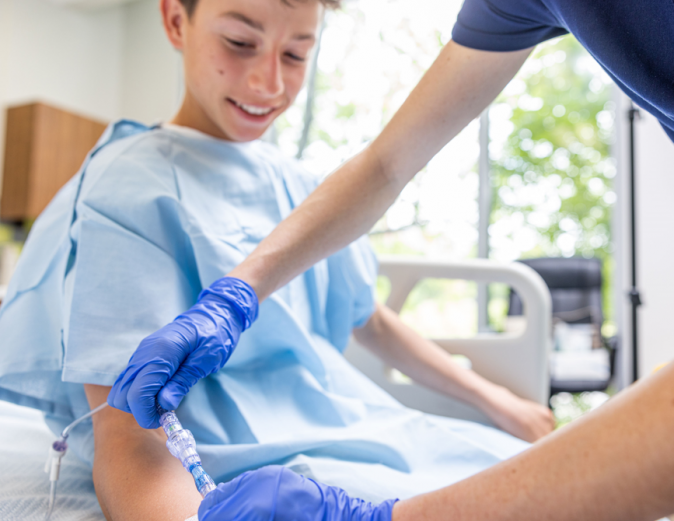
FAYETTEVILLE, Ark. - Washingtoner -- Lineus Medical, a medical device company focused on reducing IV complications, announced that the company's flagship product, SafeBreak Vascular, has received clearance from the U.S. Food and Drug Administration (FDA) for use in pediatric patients. SafeBreak Vascular has been shown in adult clinical studies to reduce IV complications with its innovative breakaway technology1.
SafeBreak Vascular is the first breakaway device cleared by the FDA and proven to reduce IV complications in adults by 44%1. It fits between the IV tubing from the IV pump and the patient's IV access point at the arm. When there is a damaging force on the line, SafeBreak separates to protect the patient's IV. This innovative feature helps prevent accidental dislodgement of the catheter, as well as other IV complications caused by damaging forces on the IV line1. This is especially important for pediatric patients who may be more active, less inclined to follow instructions, and less aware of their surroundings.
More on Washingtoner
"We are incredibly proud to receive clearance from the FDA for the use of SafeBreak Vascular with pediatric patients," said Vance Clement, CEO of Lineus Medical. "SafeBreak provides a simple, effective solution for preventing IV complications, which reduces IV restarts and additional needlesticks for patients. Now, it doesn't matter if the patient is 1 or 100 years old, SafeBreak is cleared for use on all peripheral IVs. Our goal is to have a SafeBreak on every IV line, everywhere in the world, and this additional clearance lets us help one of the most vulnerable populations that needs it the most, our kids."
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a unique, patented, break-away technology that is designed to work with all types of medical tubing. Complications with feeding tubes, IV lines, urinary catheters and any other type of medical tubing may be reduced as breakaway devices separate to protect the patient. More information about Lineus Medical can be found on the company website: www.lineusmed.com or you can follow the company on Twitter or LinkedIn.
1 Dipper Study Data on File
MKG-0194 REV 00
SafeBreak Vascular is the first breakaway device cleared by the FDA and proven to reduce IV complications in adults by 44%1. It fits between the IV tubing from the IV pump and the patient's IV access point at the arm. When there is a damaging force on the line, SafeBreak separates to protect the patient's IV. This innovative feature helps prevent accidental dislodgement of the catheter, as well as other IV complications caused by damaging forces on the IV line1. This is especially important for pediatric patients who may be more active, less inclined to follow instructions, and less aware of their surroundings.
More on Washingtoner
- Juego Studios Extends Full-Cycle Game Development & Outsourcing Capabilities to the UAE Market
- Spokane: Funding Available for Tourism and Cultural Investment Grant
- VENUS Goes Live on CATEX Exchange As UK Financial Ltd Activates The Premier Division Of The Maya Meme's League
- Our Purpose —To give "We The People" their voice back—
- Atlanta Tech Founder Seeks Clarity on Intellectual Property and Innovation Policy
"We are incredibly proud to receive clearance from the FDA for the use of SafeBreak Vascular with pediatric patients," said Vance Clement, CEO of Lineus Medical. "SafeBreak provides a simple, effective solution for preventing IV complications, which reduces IV restarts and additional needlesticks for patients. Now, it doesn't matter if the patient is 1 or 100 years old, SafeBreak is cleared for use on all peripheral IVs. Our goal is to have a SafeBreak on every IV line, everywhere in the world, and this additional clearance lets us help one of the most vulnerable populations that needs it the most, our kids."
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a unique, patented, break-away technology that is designed to work with all types of medical tubing. Complications with feeding tubes, IV lines, urinary catheters and any other type of medical tubing may be reduced as breakaway devices separate to protect the patient. More information about Lineus Medical can be found on the company website: www.lineusmed.com or you can follow the company on Twitter or LinkedIn.
1 Dipper Study Data on File
MKG-0194 REV 00
Source: Lineus Medical
0 Comments
Latest on Washingtoner
- Amicly Launches as a Safety-First Social App Designed to Help People Build Real, Meaningful Friendships
- Primeindexer Google indexing platform launched by SEO Danmark APS
- Kaltra Introduces New Downward-Spraying Distribution Technology to Boost Microchannel Evaporator Performance
- Talentica Announces Winners of Multi-Agent Hackathon 2026
- Tacoma: Applicants Sought for the Public Utility Board
- Special Alert: Undervalued Opportunity: IQSTEL (N A S D A Q: IQST) Positioned for Explosive Multi-Year Growth
- Triple-Digit Growth, Strategic N A S D A Q Uplist, Plus A Scalable Healthcare Rollout Model: Stock Symbol: CDIX
- Vesica Health Receives FDA Breakthrough Device Designation for AssureMDx
- Spokane: The Creek at Qualchan and Esmeralda Golf Courses Open March 2, 2026
- Lineus Medical's SafeBreak® Vascular Added to Alliant GPO Contract
- Cancun All Inclusive is ready for Spring Break 2026 with new Resorts, Exclusive Deals, activities and more!
- 66% of US Bankruptcies Are Medical — So Americans Are Building Businesses That Cover Healthcare Emergencies
- Ludex Partners With Certified Trading Card Association (CTCA) To Elevate Standards And Innovation In The Trading Card Industry
- Best Book Publishing Company for Aspiring Authors
- Dr. Nadene Rose Releases Moving Memoir on Faith, Grief, and Divine Presence
- Tacoma: City Council Confirms Appointment of Toni Esparza as Neighborhood & Community Services Director
- Gigasoft Solves AI's Biggest Charting Code Problem: Hallucinated Property Names
- Spokane Police Officers Involved In A Use Of Deadly Force In The 1800 Block Of West Carlisle Avenue
- ASTI Ignites the Space Economy: Powering SpaceX's NOVI AI Pathfinder with Breakthrough Solar Technology: Ascent Solar Technologies (N A S D A Q: ASTI)
- Hiring has reached a "Digital Stalemate"—Now, an ex-Google recruiter is giving candidates the answers