Trending...
- Spokane: Indian Canyon Golf Course Opens Thursday, March 12, 2026
- The Media Should Protect the Public When It Comes to Boeing — But Does It?
- Lighthouse Tech Awards Recognize Top HR Technology Providers for 2026
$NRXP Has Manufactured Multiple Commercial Lots of NRX-100 and KETAFREE™ with a Shelf Life of Three Years.
MIAMI - Washingtoner -- NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is rapidly emerging as one of the most compelling growth stories in mental-health therapeutics. With three revenue-generating treatment facilities now operating in Florida—and six expected by year-end—the company is entering 2026 with accelerating clinical operations, expanding market share, and advancing two FDA-directed regulatory pathways for ketamine-based therapeutics aimed at suicidal depression, one of the largest unmet needs in mental health.
Backed by strong clinical data, FDA Fast Track designations, and third-party revenue projections, NRXP appears positioned to capitalize on a rapidly expanding U.S. ketamine market currently estimated at $750 million, projected to reach $3.35 billion globally by 2034.
Adding to investor enthusiasm, analyst D. Boral issued a Buy rating and a $34 price target, citing NRXP's accelerating execution and diversified commercialization strategy.
A Mission Targeting One of America's Most Pressing Health Crises
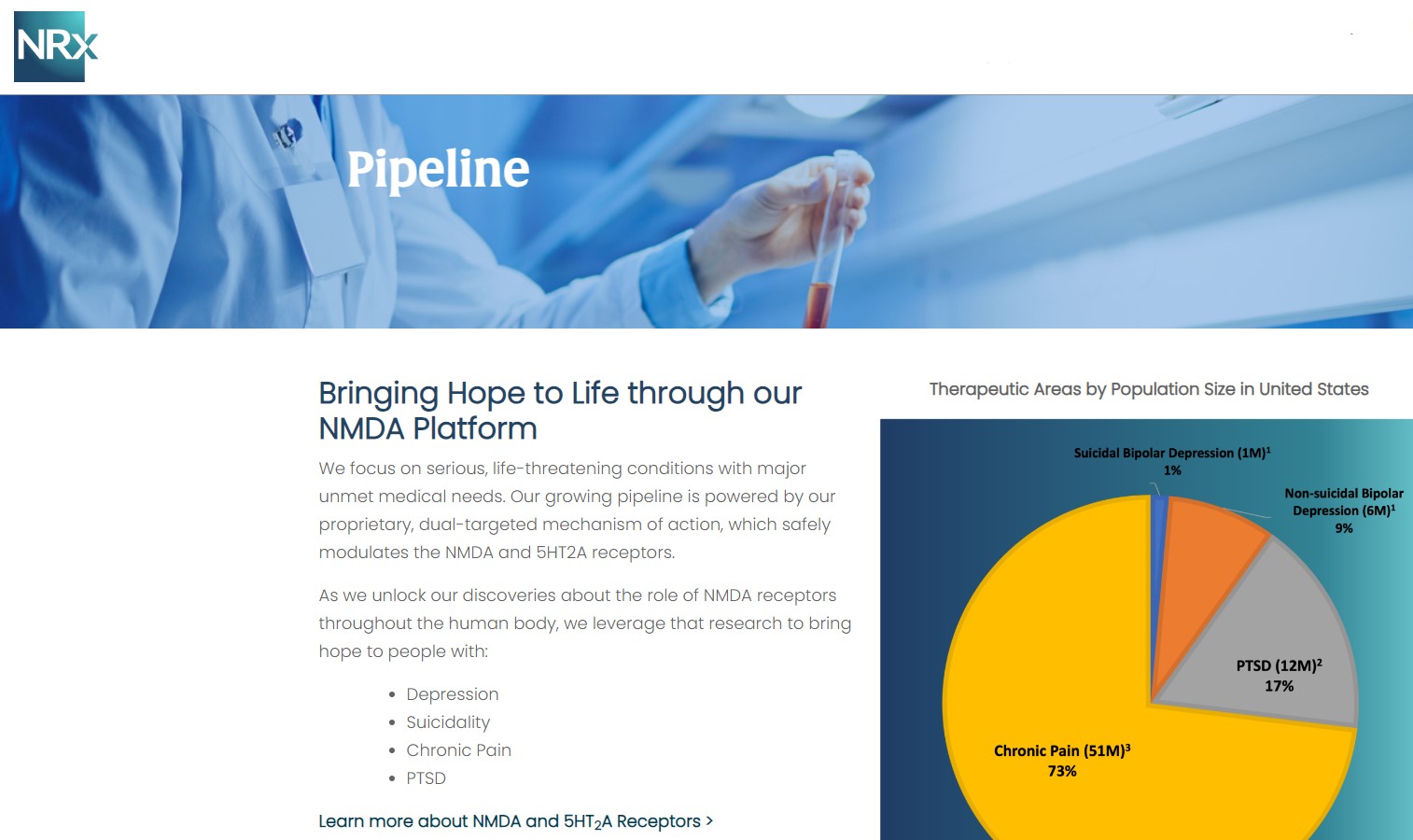
More than 13 million Americans seriously consider suicide each year (CDC). NRXP is developing next-generation therapeutics aimed directly at this crisis through its NMDA-based drug platform:
Key Pipeline Assets
NRXP is partnered with Alvogen Pharmaceuticals for the development and commercialization of NRX-101.
More on Washingtoner
A Dual-Path Strategy for NRX-100: Innovative and Generic Market Entry
NRXP's regulatory strategy is unique: it is simultaneously pursuing both an innovative NDA pathway and a generic ANDA pathway for NRX-100 and KETAFREE™.
This creates two powerful value drivers:
1. Innovative NDA for Suicidal Depression
2. ANDA for KETAFREE™ (Generic Ketamine)
With commercial lots already manufactured, NRXP is positioned for a swift commercial rollout upon approval.
HOPE Clinics: Expanding Revenue Footprint Across Florida
NRXP's HOPE Therapeutics subsidiary is advancing a scalable clinic model for ketamine and advanced TMS-based interventions.
Current & Expected Expansion
These facilities support:
Management expects increased revenue contributions from clinical operations through 2026.
Breakthrough ONE-D: First-in-Florida Deployment with Ampa Health
In November, NRXP launched patient treatment using Ampa Health's ONE-D protocol—a groundbreaking approach reporting:
ONE-D combines:
ONE-D is being deployed at HOPE clinics in Sarasota, Naples, and Fort Myers, with additional locations coming online in 2025.
More on Washingtoner
This marks a major competitive differentiator for NRXP in the mental-health treatment sector.
Financial Position: Capital Secured Through July 2026
NRXP reported securing sufficient operating capital to support its drug development programs through July 2026, complementing increasing clinical revenues.
Third-quarter results (released November 17, 2025) highlighted:
The full Q3 update and webcast are available on the NRXP investor relations site.
High-Value Licensing Opportunity
NRXP has accepted non-binding potential terms from an international pharmaceutical partner for the licensing and distribution of NRX-100. Terms include:
If finalized, this could represent a transformative revenue opportunity for the company.
Investment Outlook
With:
NRx Pharmaceuticals is attracting increased attention from both institutional and retail investors.
The recent $34 price target from D. Boral underscores growing confidence in NRXP's ability to execute on a scalable, high-margin strategy targeting one of the most underserved and urgent areas of mental health.
For More Information
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Chief Business Officer: Matthew Duffy
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Backed by strong clinical data, FDA Fast Track designations, and third-party revenue projections, NRXP appears positioned to capitalize on a rapidly expanding U.S. ketamine market currently estimated at $750 million, projected to reach $3.35 billion globally by 2034.
Adding to investor enthusiasm, analyst D. Boral issued a Buy rating and a $34 price target, citing NRXP's accelerating execution and diversified commercialization strategy.
A Mission Targeting One of America's Most Pressing Health Crises
More than 13 million Americans seriously consider suicide each year (CDC). NRXP is developing next-generation therapeutics aimed directly at this crisis through its NMDA-based drug platform:
Key Pipeline Assets
- NRX-100 (IV Ketamine):
- FDA Fast Track designation for suicidal ideation in depression, including bipolar depression.
- Multiple commercial lots produced with three-year shelf life stability.
- NDA submission expected in Q4 2025, supported by real-world patient data from more than 60,000 IV ketamine cases.
- KETAFREE™ (preservative-free ketamine):
- Pursuing approval via an ANDA (generic) pathway.
- Recently received supportive FDA correspondence confirming no major deficiencies and on track for a Q2 2026 GDUFA date.
- Addresses a ketamine market worth ~$750 million annually.
- NRX-101 (D-cycloserine + lurasidone):
- FDA-designated Investigational Breakthrough Therapy.
- Targets suicidal treatment-resistant bipolar depression, chronic pain, and potential utility in complicated UTIs.
- New real-world data suggest its active ingredient doubles the effectiveness of TMS—a potential major indication expansion.
NRXP is partnered with Alvogen Pharmaceuticals for the development and commercialization of NRX-101.
More on Washingtoner
- Peccioli Becomes New Orleans: In July 2026, the magic of jazz comes to Tuscany
- Spokane: Flags to be Lowered in Remembrance of Reverend Jesse Jackson
- $6 Million Funding Secured as Retail Expansion, Operational Streamlining, and Asset-Light Strategy Position the Company for Accelerated Growth $SOWG
- Why Your Dental Practice Ranks on Google But Still Is Not Getting New Patients
- The "Unsexy" Business Quietly Creating 130+ New Entrepreneurs Across America — From Alaska to Puerto Rico
A Dual-Path Strategy for NRX-100: Innovative and Generic Market Entry
NRXP's regulatory strategy is unique: it is simultaneously pursuing both an innovative NDA pathway and a generic ANDA pathway for NRX-100 and KETAFREE™.
This creates two powerful value drivers:
1. Innovative NDA for Suicidal Depression
- Expected completion: Q4 2025.
- Includes comparative real-world data showing IV ketamine may have faster onset and greater effect than intranasal S-ketamine.
- NRXP has applied for a Commissioner's National Priority Voucher, which could accelerate FDA review.
2. ANDA for KETAFREE™ (Generic Ketamine)
- Re-filed after FDA approved NRXP's Suitability Petition for its preservative-free formulation.
- Supportive FDA feedback in November 2025 indicated no significant deficiencies.
- A Citizen Petition filed to remove toxic preservative benzethonium chloride from ketamine products could reshape the competitive landscape.
With commercial lots already manufactured, NRXP is positioned for a swift commercial rollout upon approval.
HOPE Clinics: Expanding Revenue Footprint Across Florida
NRXP's HOPE Therapeutics subsidiary is advancing a scalable clinic model for ketamine and advanced TMS-based interventions.
Current & Expected Expansion
- 3 operational facilities in Florida
- 6 additional facilities planned by year-end
These facilities support:
- NRX-100 Expanded Access Program
- Ketamine-based clinical services
- ONE-D single-day TMS depression treatments
- Veterans, first responders, and active-duty military populations
Management expects increased revenue contributions from clinical operations through 2026.
Breakthrough ONE-D: First-in-Florida Deployment with Ampa Health
In November, NRXP launched patient treatment using Ampa Health's ONE-D protocol—a groundbreaking approach reporting:
- 87% response rate
- 72% remission
- Achieved in one day, instead of traditional 90-day TMS schedules
ONE-D combines:
- Ampa's FDA-cleared TMS device
- D-cycloserine (NRX-101 active ingredient)
- Lisdexamfetamine
ONE-D is being deployed at HOPE clinics in Sarasota, Naples, and Fort Myers, with additional locations coming online in 2025.
More on Washingtoner
- Veteran Launches GTG Energy: Nicotine-Free Pouch as Americans Rethink Addiction, Focus, and What Fuels Performance
- City of Tacoma Elevates 28-Year South African Sister City Relationship to District-Wide Partnership
- RecallSentry™ App Launch — Your Home Safety Hub — Free on iOS & Android
- Award-Winning Director Crystal J. Huang's Under-$50K Film "The Ritual House" Wins Best Horror Feature at Golden State Film Festival
- Grads aren't getting hired — here's what we're doing about it
This marks a major competitive differentiator for NRXP in the mental-health treatment sector.
Financial Position: Capital Secured Through July 2026
NRXP reported securing sufficient operating capital to support its drug development programs through July 2026, complementing increasing clinical revenues.
Third-quarter results (released November 17, 2025) highlighted:
- Progress across all clinical and regulatory objectives
- Expanded Fast Track designation for NRX-100
- EU and NIH data enhancing regulatory strategy
- Growth of the HOPE facility platform
- Enhanced IP and formulation positioning
The full Q3 update and webcast are available on the NRXP investor relations site.
High-Value Licensing Opportunity
NRXP has accepted non-binding potential terms from an international pharmaceutical partner for the licensing and distribution of NRX-100. Terms include:
- Over $300 million in milestone payments
- Tiered double-digit royalties
If finalized, this could represent a transformative revenue opportunity for the company.
Investment Outlook
With:
- Multiple clinical catalysts approaching
- Two regulatory pathways advancing for NRX-100
- FDA Breakthrough Therapy designations
- National leadership in cutting-edge TMS + D-cycloserine therapy
- Increasing real-world data support
- A growing network of revenue-producing treatment centers
- Newly manufactured commercial drug supply
- And a strong capital runway
NRx Pharmaceuticals is attracting increased attention from both institutional and retail investors.
The recent $34 price target from D. Boral underscores growing confidence in NRXP's ability to execute on a scalable, high-margin strategy targeting one of the most underserved and urgent areas of mental health.
For More Information
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Chief Business Officer: Matthew Duffy
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Financial
0 Comments
Latest on Washingtoner
- Author Ken Mora to Celebrate New Caravaggio Book Debut with Special Event at Palazzo Venezia Naples
- Matthew Sisneros Releases Raw and Unfiltered Memoir: The Devil Lost Another One — A Powerful Story of Crime, Consequence, and Redemption
- From Life to Light: Jess L. Martinez Shares a Soulful Poetry Collection That Explores What It Means to Be Human
- Lawsuit Filed Against Boeing Over Defective Seat Switch on Boeing 787
- Quadcode Acquires Significant Stake in Game 7, LLC - The Parent Company for FPFX Tech and PropAccount.com
- Danholm Collection Announces Sale of 16689 Broadwater Ave in Winter Garden, Highlighting Strong Performance in Twinwaters Community
- Strong Clinical Results for Breakthrough Liver Diagnostic Platform; ENDRA Life Sciences (N A S D A Q: NDRA) $NDRA
- 46th International Symposium On Forecasting – Dates, Venue And Speakers Announced
- Phoenix Rebellion Therapy Celebrates 10 Years Helping Utahns Overcome Trauma as Utah Faces Nation's 2nd-Highest Rate of Mental Health Challenges
- Bonavita Luxury & Portable Lavatories Announces Rebrand to Bonavita Site Solutions
- Raleigh Emerges as a Key Player in Sustainable Fashion Innovation for 2026
- Notice: Hrm Queen Laurence I Assumes Crown Control & $317q Fund. 3bn Unopoly Shares Settled. Requisition Of Buckingham Palace & Windsor Castle Final
- 13 Full Moons of Black Dandelion Convergent Voice™ An Integration of Literacy & Wellness Symposium
- Yoga Retreats, Ecstatic Dance & Spiritual App launched
- Elder Abuse Case Against Healthy Traditions Owner Raises Questions As To The Dire Reality Of Abuse Against The Last Of The Baby Boomers
- Spokane: Indian Canyon Golf Course Opens Thursday, March 12, 2026
- Federal Contract Fraud: The GUBERMAN Anomaly Exposes Boeing–ANAB Collusion in Contract 19AQMM18R0131
- Simpalm Staffing Services Launched its Refreshed Website for Remote Staffing Services
- Claude Riveloux Review 2026: How the $10B Fund Manager Dispels 'Scam' Rumors Through Education
- Pure Energy Electrical Services, LLC Announces Strong Start to 2026, Reinforcing Customer-First Electrical Service Across Northeast Florida